41 fda approved health claims on food labels
FDA Label Claims For Low Carb Diets However, the U.S. Food and Drug Administration (FDA) regulate claims that are used on food and supplements labels, and it has categorized the applications into three categories as defined by the FDA statute: health claims, nutrient content claims, and structure/function claims. · Health claims: - These are statements that describe a ... Authorized Health Claims That Meet the Significant ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Factual Food Labels: Health Claims - University of Texas ... According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims. In 1990, the Nutrition ...
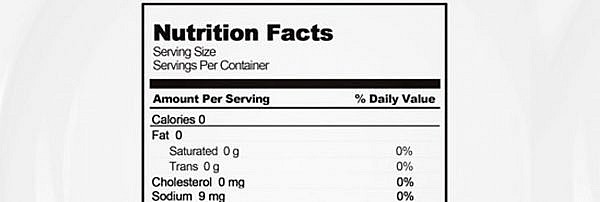
Fda approved health claims on food labels
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". 21 CFR § 101.14 - Health claims: general requirements ... (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes ... What are some examples of an FDA health claim on a food label? Also asked, what health claims are allowed on food labels? Approved Health Claims Calcium, Vitamin D, and Osteoporosis. Dietary Lipids (Fat) and Cancer. Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease. Dietary Non-cariogenic Carbohydrate Sweeteners and Dental Caries.
Fda approved health claims on food labels. Qualified Health Claims - FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim" ch2 Flashcards - Quizlet health claim on a food label that has been approved based on emerging but not well-established evidence of a relationship between a food, food component, or dietary supplement and reduced risk of a disease or health-related condition; must be accompanied by an explanatory statement to avoid misleading customers Food labelling and packaging: Nutrition, health claims and ... Food and drink labelling and packaging regulations - what you must show, warnings, health and organic labels and packaging standards. Food labelling and packaging: Nutrition, health claims and ... A Guide to FDA Regulation of Food Labeling Claims ... Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
FDA approves cardiovascular health claim on certain oil ... Regardless, the United States Food and Drug Administration has announced that it will allow all olive oil bottles to carry a new "qualified health claim" on their labels. With this, manufacturers... Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if... CFR - Code of Federal Regulations Title 21 - Food and Drug ... (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes, characterizes the ... Health Claims On Your Food - Tufts Health & Nutrition Letter Although some people may call any type of health or nutrition message on a food package a "health claim," this isn't really consistent with the FDA's definition. Packaged food and beverage labels may carry four general types of claims, which include health claims, qualified health claims, structure/function claims and nutrient content claims.
ABC's of Health Claims - WebMD Become familiar with the FDA approved claims and seek to fill your basket with foods we know are good for us. When the claims ratings start showing up on packages, stick with the A's and B's. Read... Understanding Food Labels | The Nutrition Source | Harvard ... The Nutrition Labeling and Education Act of 1990 regulates these health claims, which must undergo review by the FDA through a petition process. The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and ... The FDA has approved health claims on food labels for all ... The FDA has approved health claims on food labels for all of the following conditions except: _____ asked Jul 18, 2017 in Nutritional Science by Zeanique a. osteoporosis b. heart disease c. tooth decay d. arthritis e. all of the above are approved health claims advanced-nutrition 0 votes More questions like this Food Packaging Claims - American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
Nutrient Claims on Food Labels | Home & Garden Information ... Health claims, which the FDA must authorize, describe a relationship between a nutrient or food and a disease or health-related condition. If a claim names a specific disease risk, there is substantial scientific evidence that the food product may help protect against the disease in the context of a healthy diet.
Nutrient Content Claims - FDA Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
5 Things to Know About Triclosan | FDA Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332)
Health Claims on Food Labels | LegalMatch Health claims must be approved by the Food and Drug Administration (FDA) before the manufacturer is allowed to put the claim on one of their food products . There are two ways of obtaining FDA approval: First, the manufacturer can come up with a health claim based on independent scientific studies and evidence.
Qualified Health Claims | FDA - U.S. Food and Drug ... Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
health claims and food labels Flashcards | Quizlet GRAS, EAFUS, nutrition labeling and education act, DSHEA, food and drug modernization act, public health security and bioterrorism preparedness response act GRAS generally recognized as safe. dont need a formal premarket review if added to foods. safety established by long history of food. know nature, intended use, and safety of product.
Health Claims on Food Labels: LabelCalc - FDA Compliant Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA).
EU Register of nutrition and health claims made on foods ... Health claims submitted as Article 13(1) 'function claims' (8 Kb) but that do not qualify as such. Health claims not related to human health (6 Kb) which cannot consequently be used on foods. Health claims for combinations of substances (7 Kb) where health claims are already authorised for some of the individual substances.
Structure/Function Claims | FDA - U.S. Food and Drug ... If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not...
FDA Label Search - Food and Drug Administration The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies described in monographs.

Ishigaki Glutathione Now FDA Approved | Dear Kitty Kittie Kath- Top Lifestyle, Beauty, Mommy ...
Label Claims for Conventional Foods and Dietary ... there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...

Factual Food Labels: Health Claims - UT Austin 100% Online Master of Science in Nutritional Science
FDA slams Delta-8 THC sellers over claims FDA sent warning letters to five companies marketing Delta-8 THC products, several of which also have CBD product lines. In every case, the Agency came down hard on the disease claims being make ...
FDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20, extralabel use of an approved human drug in a food-producing animal is not permitted if an animal drug approved for use in food-producing animals can be used in ...
What are some examples of an FDA health claim on a food label? Also asked, what health claims are allowed on food labels? Approved Health Claims Calcium, Vitamin D, and Osteoporosis. Dietary Lipids (Fat) and Cancer. Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease. Dietary Non-cariogenic Carbohydrate Sweeteners and Dental Caries.








Post a Comment for "41 fda approved health claims on food labels"